Research overview
Our Research
The phenomenon of phase separation describes the social context in which molecules interact in our cells.
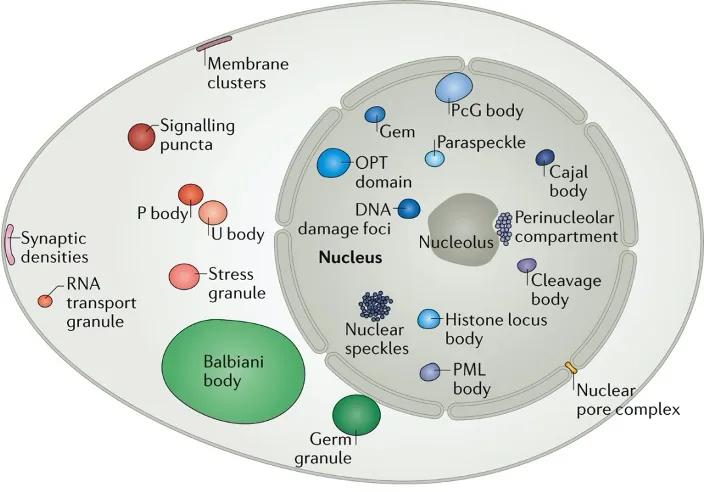
Studying the social interactions of molecules inside the cell
Consider a village where the people in it perform different tasks in different locations. They might work in the bakery, the construction site, or the power plant. In a similar way, many proteins and nucleic acids in a cell are also located in distinct compartments, where they perform diverse functions. However, most work in cells has focused on the activity and function of individual molecules rather than their collective properties in compartments.
Taking an analogy from our village, it would be similar to understanding the behaviour of individuals, without understanding the rules that govern society. Unexpectedly, recent advances show that the mechanisms by which compartments assemble may hold the key to explaining some of the biggest open questions in biology, paving the way for a revolution in our understanding of cellular physics.
The Hyman lab studies how the formation of liquid phases within a liquid cytoplasm impacts the formation of membraneless compartmentalisation of macromolecules inside living cells. Estimates suggest that at least 30% of proteins in the nucleus can be in such dynamic compartments. Stemming from our work on C. elegans in 2009, observations over the last decade have shown that many non-membrane compartments have liquid-like properties (Banani et al. 2017). The liquid-like nature of condensates is ascertained using microscopy in cells by observing fusion events, round shape, rapid diffusion of components, and a predictable response to changes in thermodynamic parameters such as temperature. Phase separation phenomena can be used to describe the formation of P granules (Brangwynne et al. 2009; Fritsch et al. 2021), nucleoli (Brangwynne et al. 2011), stress granules and centrosomes (Patel et al., 2015; Woodruff et al., 2017) and are linked to noise buffering in cells (Klosin et al., 2020). Pioneering transcription factors can condense on DNA on specific sequences in vitro (Morin et al., 2022).
To capture the role of soft matter physics in describing these compartments, we and others have termed them biomolecular condensates (Banani et al. 2017, Shin et al. 2017).
Manipulating liquid like properties inside cells requires the ability to manipulate phase properties using mutagenesis. To start analyzing the molecular grammar of condensate formation we studied proteins of the FUS family and found that multivalent interactions between arginines and tyrosines play an important role (Wang et al., 2018). Below the saturation concentration FUS forms clusters, which size and abundance can grow. Above saturation concentration these clusters grow through macroscopic phase separation in vitro (Kar et al., 2022)
Importantly, many proteins that form condensates in cells, such as FUS, TAU and TDP-43 are as well found in protein aggregates linked to neurodegenerative diseases like ALS and Alzheimer (Patel et al., 2015; Hernandez-Vega et al., 2017; Portz et al., 2021), One of the future challenges will be to understand why condensates can transform into irreversible aggregates in cells.