New work from the Hyman and Alberti labs uncovers an important role for chaperone proteins in preventing aberrant phase transitions in stress granules. See a brief synopsis below and read the full paper online. Congratulations to all of the authors on this work!
Synopsis
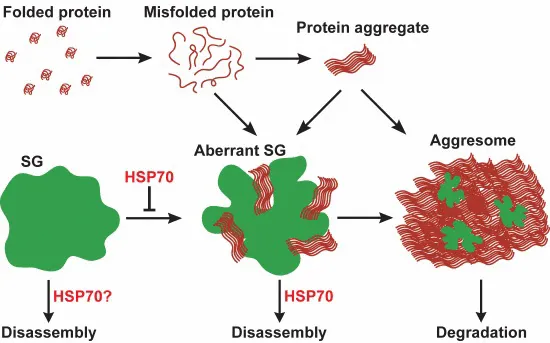
The presence of misfolded protein in stress granules alters their dynamic state and induces a phase transition. This process is counteracted by chaperones and autophagy, acting as a stress granule quality control system.
- Misfolded proteins have a tendency to aggregate in stress granules (SGs).
- Misfolded proteins promote a conversion of SGs into an aberrant solid‐like state.
- Chaperones prevent the formation of aberrant SGs and promote SG disassembly.
- Persistent aberrant SGs are targeted to the aggresome for degradation.
An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. Mateju D, Franzmann TM, Patel A, Kopach A, Boczeck EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S. The EMBO Journal. (2017) e201695957. [FullText]