Congratulations to Jeff Woodruff, Oliver Wueseke, and colleagues on their recent publication in Science! This work describes a novel in vitro system for studying regulated assembly of the pericentriolar matrix (PCM), revealing that networks of the protein SPD-5 can polymerize into interconnected, porous networks that specifically recruit PCM proteins. Stay tuned for a video abstract, coming soon!
Centrosomes. Regulated assembly of a supramolecular centrosome scaffold in vitro. Woodruff JB, Wueseke O, Viscardi V, Mahamid J, Ochoa SD, Bunkenborg J, Widlund PO, Pozniakovsky A, Zanin E, Bahmanyar S,Zinke A, Hong SH, Decker M, Baumeister W, Andersen JS, Oegema K, Hyman AA. _Science. 2_015 May 15;348(6236):808-12. (Please visit our Publications page for the referrer links to the free full text and PDF.)
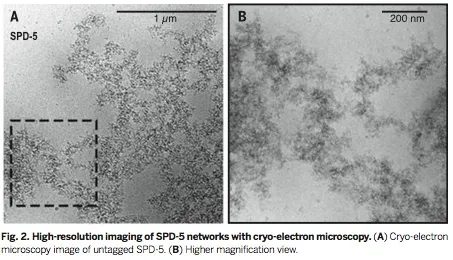
Abstract: The centrosome organizes microtubule arrays within animal cells and comprises two centrioles surrounded by an amorphous protein mass called the pericentriolar material (PCM). Despite the importance of centrosomes as microtubule-organizing centers, the mechanism and regulation of PCM assembly are not well understood. In Caenorhabditis elegans, PCM assembly requires the coiled-coil protein SPD-5. We found that recombinant SPD-5 could polymerize to form micrometer-sized porous networks in vitro. Network assembly was accelerated by two conserved regulators that control PCM assembly in vivo, Polo-like kinase-1 and SPD-2/Cep192. Only the assembled SPD-5 networks, and not unassembled SPD-5 protein, functioned as a scaffold for other PCM proteins. Thus, PCM size and binding capacity emerge from the regulated polymerization of one coiled-coil protein to form a porous network.